Researchers are exploring a novel approach to combat amyotrophic lateral sclerosis (ALS), a devastating neurodegenerative disease, using CAR T-cell therapy. This experimental treatment aims not to cure ALS, but to significantly slow its relentless progression, offering a lifeline to patients facing a grim prognosis of just two to five years post-diagnosis.
The Problem with ALS: Inflammation and Rogue Immune Cells
ALS, also known as Lou Gehrig’s disease, results from the death of motor neurons, the cells responsible for controlling voluntary muscle movement. While genetic mutations account for only a small percentage of cases (5-10%), the vast majority of ALS diagnoses are sporadic, with unknown causes and no effective treatments. However, accumulating evidence points to inflammation in the brain as a major driver of motor neuron loss.
Specifically, immune cells called microglia appear to become overactive in ALS patients, mistakenly attacking and pruning too many synapses – the connections between neurons – ultimately contributing to neuronal death. These “damage-amplifying microglia” display a unique protein marker, uPAR, on their surface, providing a potential target for intervention.
How CAR T-Cell Therapy Works
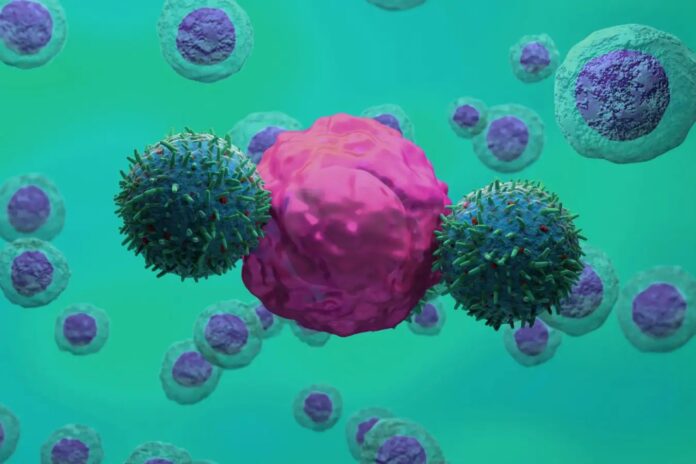
The key to this approach lies in CAR T-cells, genetically engineered immune cells already proven effective in treating certain cancers. Researchers modify these cells to specifically recognize and kill cells displaying uPAR, effectively eliminating the rogue microglia without harming healthy neurons.
In lab tests, CAR T-cells targeting uPAR successfully eliminated the damaging microglia, suggesting the therapy could halt further neuronal loss. While it cannot restore lost motor neurons, it represents a significant step towards stabilizing the condition.
Trials and Potential Broader Applications
Animal trials are underway using mice engineered to develop ALS. Promising results could expedite human trials, given the disease’s severity and the lack of existing treatments. The urgency of the situation may lead to faster regulatory approvals if the initial findings hold true.
Furthermore, experts believe that damage-amplifying microglia may play a role in other neurodegenerative conditions, like certain forms of dementia. This suggests the same CAR T-cell approach could have broader applications beyond ALS, offering a potential new strategy for slowing down these debilitating diseases.
“The evidence for immune dysfunction in ALS is mounting… This seems a very promising and interesting approach.” – Ammar Al-Chalabi, King’s College London.
This research represents a critical shift in how we think about treating ALS, and potentially other neurodegenerative conditions. By targeting the underlying inflammatory processes, rather than simply managing symptoms, CAR T-cell therapy offers a realistic path toward extending quality of life for patients facing otherwise devastating outcomes.